COVID-19 Pre-Exposure Prophylaxis
Evusheld (Tixagevimab/Cilgavimab): The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of the unapproved product EVUSHELD (tixagevimab co-packaged with cilgavimab) for the pre-exposure prophylaxis of coronavirus disease 2019 (COVID-19) in adults and pediatric individuals (12 years of age and older weighing at least 40 kg). Evusheld EUA.
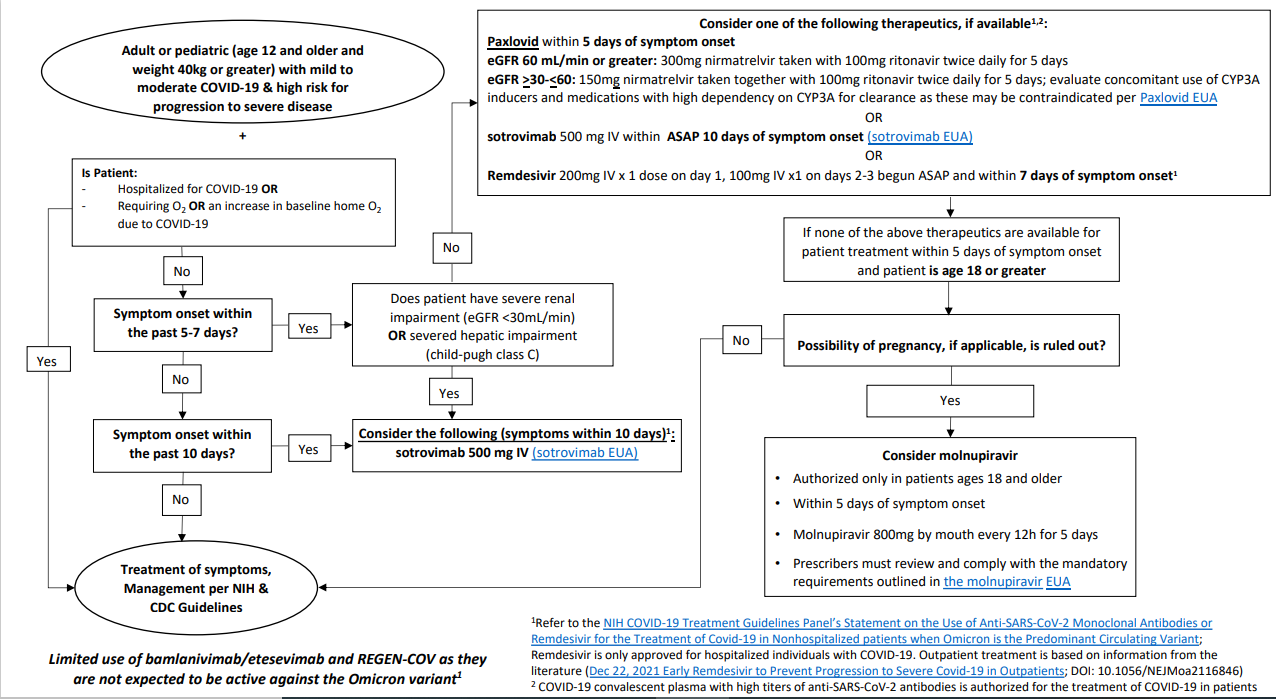
COVID-19 Therapies/Treatments
The federal government will allocate emergency use authorization (EUA) COVID-19 treatments to California, and CDPH will allocate to jurisdictions based on new COVID-19 cases and an equity measure. Get a side-by-side comparison of Therapeutics.
Molnupiravir: On December 23, 2021, the U.S. FDA issued a EUA for Merck's Molnupiravir for outpatient treatment of mild-to-moderate coronavirus disease (COVID-19) in adults (18 years of age and older). Molnupiravir is authorized for use in situations where other FDA-authorized treatments for COVID-19 are inaccessible or are not clinically appropriate, along with considering the potential risks/benefits to prescribing Molnupiravir. Molnupiravir EUA
Paxlovid: On December 22, 2021, the U.S. FDA issued an EUA for Pfizer's Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms). Paxlovid EUA | Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
Remdesivir: The FDA has also approved the antiviral drug Veklury (remdesivir) for adults and certain pediatric patients (weighing 8 pounds (3.5 kg) to less than 88 pounds (40 kg) or children less than 12 years of age weighing at least 8 pounds (3.5 kg)) with positive results of direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral testing, who are hospitalized, or not hospitalized and have mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death. Remdesivir EAU
Bebtelovimab: The FDA approved Bebtelovimab, a monoclonal antibody, on February 11, 2022, and is a monoclonal antibody treatment, injected intraveneously. The EUA for bebtelovimab is for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms, which is about 88 pounds) with a positive COVID-19 test, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options approved or authorized by the FDA are not accessible or clinically appropriate. Bebtelovimab EAU
Previously, EUAs had been issued for Bamlanivimab/Etesevimab and Casirivimab/Imdevimab, but have since been revoked as they are proven to be ineffective against the dominant strain - Omicron variant.
*Molnupiravir should not be given to patients who are or may become pregnant. Furthermore, patients should be advised any attempt at conception should be paused while taking this medication.
COVID-19 Test to Treat Map and Program Information
- A new web-based COVID-19 Test to Treat Locator Map is now available to make it easier to find Test to Treat locations. In the Test to Treat program, people are able to get tested and – if they are positive and treatments are appropriate for them – receive a prescription from a health care provider, and then have their prescription filled all at one location.
Federal Response to COVID-19: Therapeutics Clinical Implementation Guide